Outer Shell Of An Atom
ii.v: Arrangement of Electron (Beat out Model)
- Folio ID
- 15564
An electron trounce is the outside part of an atom effectually the diminutive nucleus. It is a group of atomic orbitals with the same value of the primary quantum number \(due north\). Electron shells have one or more electron subshells, or sublevels. The name for electron shells comes from the Bohr model, in which groups of electrons were believed to go around the nucleus at certain distances, so that their orbits formed "shells".
Introduction
What more than could we desire to know nearly the structure of an atom? We know that atoms incorporate positively and negatively charged particles, and that the number of these charges in each cantlet is different for each element. We also know that the positive charges are full-bodied in a tiny nucleus, and that the electrons motion around the nucleus in a space that is much, much larger than the nucleus.
Even so, some of the most important questions nosotros asked in the previous Concept Development Study are notwithstanding unanswered. Remember that we saw that carbon and nitrogen have very similar atomic masses. At present we tin add that these elements accept very similar atomic numbers, so their atoms have similar numbers of protons and electrons. But carbon and nitrogen are, in most chemical and physical ways, very different. Similarly, some elements like sodium and potassium accept very different diminutive numbers but have quite similar chemical and physical properties. It seems that comparing the backdrop of two unlike atoms is not very easy to understand just from comparing the numbers of protons and electrons the atoms incorporate.
To continue to empathise the answers to these questions, we need fifty-fifty more than item about the structure of each type of cantlet. Knowledge of the electron configuration of unlike atoms is useful in agreement the structure of the periodic table of elements. The concept is too useful for describing the chemical bonds that hold atoms together.
Shells
An electron shell may exist thought of as an orbit followed by electrons around an atom nucleus. Because each shell can comprise but a fixed number of electrons, each vanquish is associated with a particular range of electron free energy, and thus each shell must fill up completely before electrons tin be added to an outer shell. The electrons in the outermost shell determine the chemical backdrop of the atom (see Valence shell). For an caption of why electrons exist in these shells see electron configuration.
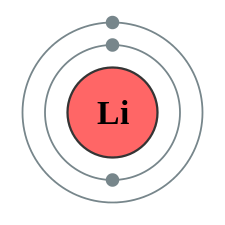
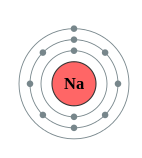
Figure \(\PageIndex{1}\): A trounce Diagram of lithium (left) and Sodium (right)
The electron shells are labeled M, L, Grand, North, O, P, and Q; or 1, ii, 3, 4, 5, half dozen, and 7; going from innermost vanquish outwards. Electrons in outer shells have higher boilerplate free energy and travel farther from the nucleus than those in inner shells. This makes them more than important in determining how the atom reacts chemically and behaves every bit a conductor, considering the pull of the cantlet's nucleus upon them is weaker and more hands broken. In this way, a given chemical element'south reactivity is highly dependent upon its electronic configuration.
Valence Electrons
The valence shell is the outermost shell of an atom in its uncombined state, which contains the electrons near probable to account for the nature of any reactions involving the atom and of the bonding interactions it has with other atoms. Valence electrons are electrons that are associated with an atom, and that can participate in the germination of a chemical bond; in a single covalent bail, both atoms in the bond contribute ane valence electron in order to course a shared pair. The presence of valence electrons tin make up one's mind the element's chemical properties and whether it may bond with other elements: For a main group element, a valence electron tin only be in the outermost electron shell.
An cantlet with a airtight shell of valence electrons tends to be chemically inert. An atom with one or two valence electrons more than a closed shell is highly reactive, considering the extra valence electrons are easily removed to grade a positive ion. An atom with one or two valence electrons fewer than a closed shell is also highly reactive, considering of a tendency either to proceeds the missing valence electrons (thereby forming a negative ion), or to share valence electrons (thereby forming a covalent bond).
Like an electron in an inner beat, a valence electron has the ability to blot or release energy in the form of a photon. An energy proceeds can trigger an electron to motion (jump) to an outer shell; this is known every bit atomic excitation. Or the electron can even break complimentary from its associated cantlet's valence shell; this is ionization to course a positive ion. When an electron loses energy (thereby causing a photon to be emitted), then information technology tin motility to an inner crush which is non fully occupied.
The number of valence electrons of an chemical element tin be determined past the periodic tabular array group (vertical column) in which the element is categorized. With the exception of groups 3–12 (the transition metals), the units digit of the grouping number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular column.
| Periodic table group | Valence electrons |
|---|---|
| Group ane (I) (alkali metals) | one |
| Group ii (II) (element of group i world metals) | 2 |
| Groups iii-12 (transition metals) | 2* |
| Group thirteen (III) (boron group) | three |
| Group 14 (4) (carbon group) | four |
| Group xv (V) (pnictogens) | 5 |
| Grouping xvi (VI) (chalcogens) | 6 |
| Group 17 (Vii) (halogens) | vii |
| Group xviii (Eight or 0) (noble gases) | 8** |
* The general method for counting valence electrons is generally non useful for transition metals.
** Except for helium, which has merely two valence electrons.
Contributors
- Connections (John Hutchinson)
- Wikipedia
Outer Shell Of An Atom,
Source: https://chem.libretexts.org/Courses/Sacramento_City_College/SCC:_Chem_309_-_General_Organic_and_Biochemistry_%28Bennett%29/Text/02._Atomic_Structure/2.5:_Arrangement_of_Electron_%28Shell_Model%29
Posted by: mansfieldsperve.blogspot.com


0 Response to "Outer Shell Of An Atom"
Post a Comment